By Tenzin Choephel*
Covid-19 has pretty much forced every other person on this planet to stay home twenty-four-seven in the hope of flattening the epidemic curve. This has been frustrating to me personally as it has been to many of you. While complaints of boredom and stress as a result of spending days and weeks within the claustrophobic confines of ones homes are completely understandable, these times also provide an opportunity to explore the mysterious corners and alleys of your homes that otherwise remain unexplored or unnoticed during normal times. I have discovered one such corners to be a few boxes of books in my storage that I have collected over the years; some of the books I must have half-read at some point in the hope of finishing it at some later time. One such books happened to be "Ideas and Opinions", a collection of scientific and non-scientific essays by Albert Einstein. Sitting in the back deck with a cup of tea on this beautiful April afternoon, I flipped through the pages of this book and came across this short essay that Einstein wrote in 1946. He explains in his own words the meaning of the equation that not only rocked the scientific world but also captured the imagination of the popular culture at the time. To those of you who are technically inclined, you will appreciate the simplicity and elegance of the way it is explained. To those who are not so technically inclined, you will still be able to appreciate the basic meaning of his equation... hopefully.
E = mc2
By Albert Einstein from Science Illustrated, New York, April, 1946
IN ORDER TO UNDERSTAND the law of the equivalence of mass and energy, we must go back to two conservation or “balance” principles which, independent of each other, held a high place in pre-relativity physics. These were the principle of the conservation of energy and the principle of the conservation of mass. The first of these, advanced by Leibnitz as long ago as the seventeenth century, was developed in the nineteenth century essentially as a corollary of a principle of mechanics.
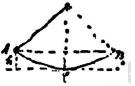
Drawing from Dr. Einstein’s manuscript
Consider, for example, a pendulum whose mass swings back and forth between the points A and B. At these points the mass m is higher by the amount h than it is at C, the lowest point of the path (see drawing). At C, on the other hand, the lifting height has disappeared and instead of it the mass has a velocity v. It is as though the lifting height could be converted entirely into velocity, and vice versa. The exact relation would be expressed as
![]()
with g representing the acceleration of gravity. What is interesting here is that this relation is independent of both the length of the pendulum and the form of the path through which the mass moves.
The significance is that something remains constant throughout the process, and that something is energy. At A and at B it is an energy of position, or “potential” energy; at C it is an energy of motion, or “kinetic” energy. If this concept is correct, then the sum
![]()
must have the same value for any position of the pendulum, if h is understood to represent the height above C, and v the velocity at that point in the pendulum's path. And such is found to be actually the case. The generalization of this principle gives us the law of the conservation of mechanical energy. But what happens when friction stops the pendulum?
The answer to that was found in the study of heat phenomena. This study, based on the assumption that heat is an indestructible substance which flows from a warmer to a colder object, seemed to give us a principle of the “conservation of heat.” On the other hand, from time immemorial it has been known that heat could be produced by friction, as in the fire-making drills of the Indians. The physicists were for long unable to account for this kind of heat “production.” Their difficulties were overcome only when it was successfully established that, for any given amount of heat produced by friction, an exactly proportional amount of energy had to be expended. Thus did we arrive at a principle of the “equivalence of work and heat.” With our pendulum, for example, mechanical energy is gradually converted by friction into heat.
In such fashion the principles of the conservation of mechanical and thermal energies were merged into one. The physicists were thereupon persuaded that the conservation principle could be further extended to take in chemical and electromagnetic processes—in short, could be applied to all fields. It appeared that in our physical system there was a sum total of energies that remained constant through all changes that might occur.
Now for the principle of the conservation of mass. Mass is defined by the resistance that a body opposes to its acceleration (inert mass). It is also measured by the weight of the body (heavy mass). That these two radically different definitions lead to the same value for the mass of a body is, in itself, an astonishing fact. According to the principle—namely, that masses remain unchanged under any physical or chemical changes—the mass appeared to be the essential (because unvarying) quality of matter. Heating, melting, vaporization, or combining into chemical compounds would not change the total mass.
Physicists accepted this principle up to a few decades ago. But it proved inadequate in the face of the special theory of relativity. It was therefore merged with the energy principle—just as, about 60 years before, the principle of the conservation of mechanical energy had been combined with the principle of the conservation of heat. We might say that the principle of the conservation of energy, having previously swallowed up that of the conservation of heat, now proceeded to swallow that of the conservation of mass—and holds the field alone.
It is customary to express the equivalence of mass and energy (though somewhat inexactly) by the formula E = mc2, in which c represents the velocity of light, about 186,000 miles per second. E is the energy that is contained in a stationary body; m is its mass. The energy that belongs to the mass m is equal to this mass, multiplied by the square of the enormous speed of light—which is to say, a vast amount of energy for every unit of mass.
But if every gram of material contains this tremendous energy, why did it go so long unnoticed? The answer is simple enough: so long as none of the energy is given off externally, it cannot be observed. It is as though a man who is fabulously rich should never spend or give away a cent; no one could tell how rich he was.
Now we can reverse the relation and say that an increase of E in the amount of energy must be accompanied by an increase of
![]()
in the mass. I can easily supply energy to the mass—for instance, if I heat it by 10 degrees. So why not measure the mass increase, or weight increase, connected with this change? The trouble here is that in the mass increase the enormous factor c2 occurs in the denominator of the fraction. In such a case the increase is too small to be measured directly; even with the most sensitive balance.
For a mass increase to be measurable, the change of energy per mass unit must be enormously large. We know of only one sphere in which such amounts of energy per mass unit are released: namely, radioactive disintegration. Schematically, the process goes like this: An atom of the mass M splits into two atoms of the mass M′ and M″, which separate with tremendous kinetic energy. If we imagine these two masses as brought to rest—that is, if we take this energy of motion from them—then, considered together, they are essentially poorer in energy than was the original atom. According to the equivalence principle, the mass sum M′ M″ of the disintegration products must also be somewhat smaller than the original mass M of the disintegrating atom—in contradiction to the old principle of the conservation of mass. The relative difference of the two is on the order of one-tenth of one percent.
Now, we cannot actually weigh the atoms individually. However, there are indirect methods for measuring their weights exactly. We can likewise determine the kinetic energies that are transferred to the disintegration products M′ and M″. Thus it has become possible to test and confirm the equivalence formula. Also, the law permits us to calculate in advance, from precisely determined atom weights, just how much energy will be released with any atom disintegration we have in mind. The law says nothing, of course, as to whether—or how—the disintegration reaction can be brought about.
What takes place can be illustrated with the help of our rich man. The atom M is a rich miser who, during his life, gives away no money (energy). But in his will he bequeaths his fortune to his sons M′ and M″, on condition that they give to the community a small amount, less than one thousandth of the whole estate (energy or mass). The sons together have somewhat less than the father had (the mass sum M′ M″ is somewhat smaller than the mass M of the radioactive atom). But the part given to the community, though relatively small, is still so enormously large (considered as kinetic energy) that it brings with it a great threat of evil. Averting that threat has become the most urgent problem of our time.
* Sr. Aerospace Engineer at Pratt & Whitney, USA

